Support services for importing medical devices for Russia
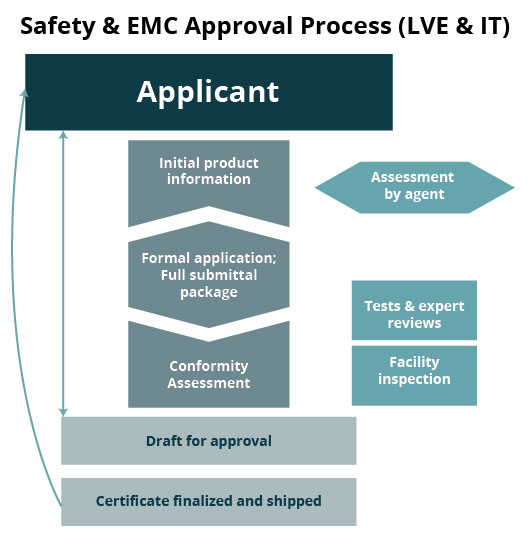
Safety & EMC Certification & Registration Process
- Advisory services for classification and product range grouping to different classes of medical devices
- Testing services (toxicology, safety tests, EMC and clinical trials) are performed by authorized institutions in Russia and exclusive testing partners
- Translation services
- Preparation of complete and sufficient Design Dossier (submittal package - Technical File) for successful registration
- Coordination services for application submission, testing and receipt of Registration Certificate
- Certificate and Declaration of Conformity (GOST R) and Custom Union Technical Regulation
All Rights Reserved 2026, ciscert.com - Admin Login | Alt Media Studios




